

When atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed. The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x 10-19 coulombs. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z. This electric charge generated on the ion is known as Ionic charge. Silver is a chemical element with atomic number 47 which means there are 47 protons in its nucleus. If more than one of a particular polyatomic ion is needed to balance the charge, the entire formula for the polyatomic ion must be enclosed in parentheses, and the numerical subscript is placed outside the parentheses. Ionic charge: When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). The rule for constructing formulas for ionic compounds containing polyatomic ions is the same as for formulas containing monatomic (single-atom) ions: the positive and negative charges must balance. A few elements have symbols derived from their Latin names as given in the table below.\): Some Polyatomic Ions Name Many elements have their symbol derived from either the first letter or the first two letters of their names.

At the very bottom is the name of the element (e.g., hydrogen). When we galvanize (zinc treat) a nail or some other bit of steel, a little cadmium comes along for the ride.
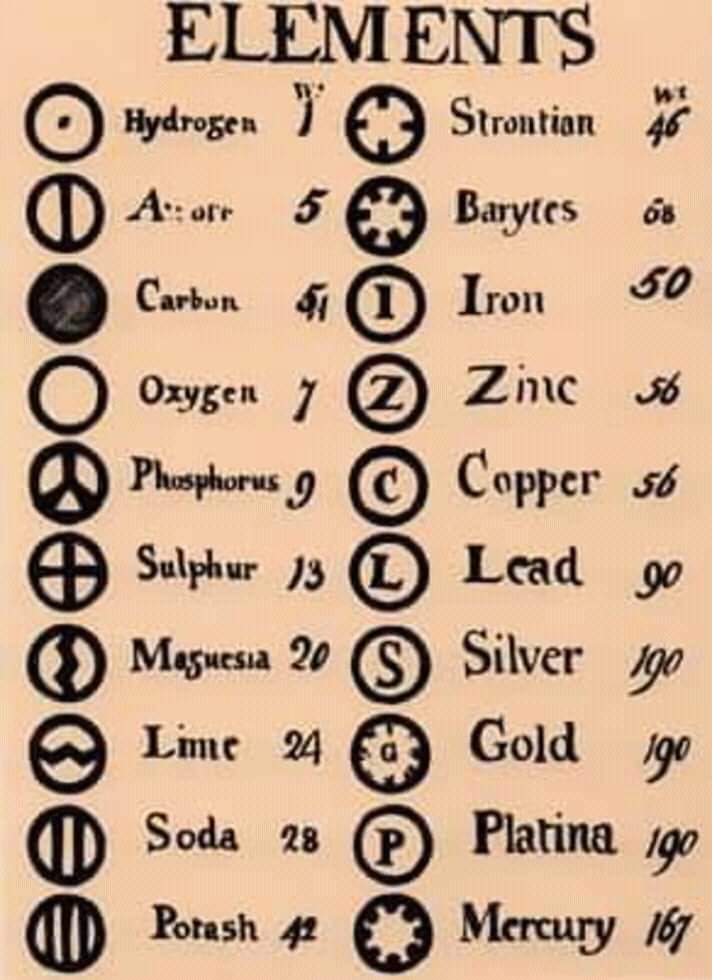
In the environment it is distributed nearly everywhere we find zinc and therefore when we mine zinc, we consequently mine cadmium. Below is the relative atomic mass, as calculated for the isotopes found naturally on Earth. Cadmium sits right below zinc on the periodic table and therefore shares many of its same chemical properties. The basic particle that constitutes a chemical element is the atom, and each chemical element is distinguished by the number of protons in the nuclei of its atoms, known as its atomic number. These individual element summary pages contain a lot of additional. When exploring the table or list views on this page, please note the links to dedicated pages for each element. In the middle is the letter symbol for the element (e.g., H). A chemical element is a chemical substance that cannot be broken down into other substances. PubChem is providing this periodic table page in order to help navigate abundant chemical element data available in PubChem. At the upper left is the atomic number, or number of protons. The names and symbols of the elements are shown in the periodic table. Image showing the 'anatomy' of a periodic table entry. In the periodic table, the vertical columns are called groups and the horizontal rows are called periods. You must write the chemical symbol of sodium as Na, not as NA, na or nA. An atom of an element is denoted by this symbol. Periodic Table of Elements - The periodic table is the tabular arrangement of all the chemical elements on the basis of their respective atomic numbers. Thus, in the standard notation, 1 1 H refers to the simplest isotope of hydrogen and 235 92 U to an isotope of uranium widely used for nuclear power. Each element has a chemical symbol that is unique to it. The specification of Z, A, and the chemical symbol (a one- or two-letter abbreviation of the element’s name, say Sy) in the form A Z Sy identifies an isotope adequately for most purposes. Cations and anions When a neutral atom loses one or more electrons, the total number of electrons decreases while the number of protons in the nucleus remains the same. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. The resulting charged species is called an ion. The symbol is the short form or abbreviated name of the element. Element Argon (Ar), Group 18, Atomic Number 18, p-block, Mass 39.95. Scientists have adopted certain conventions regarding the chemical symbols for various elements. What are the Latin Names of Chemical Elements? Chemistry : Elements and Chemical Symbols - Latin Names


 0 kommentar(er)
0 kommentar(er)
